New collaboration brings hands-on tour of unit-level traceability in injectable drug manufacturing to CPHI 2025
Frankfurt, Germany– October 15, 2025: ten23 health, the human-centric and sustainable strategic CDMO partner of choice for the pharmaceutical industry and biotech start-ups, BD (Becton, Dickinson and Company), together with Harro Höfliger, and Crypto Pharma will jointly present an end-to-end demonstration of RFID-enabled syringe traceability at CPHI 2025, running October 28–30 in Frankfurt. The BD iDFill™ RFID Integration Tour will allow registered attendees to experience a full production cycle — from filling to final device assembly — across four booths, highlighting how unit-level identification can improve safety, quality, and compliance in injectable drug production.
“We are excited about the experience provided to CPHI attendees, the partnership with BD, Harro Höfliger and Cryptopharma, about the possibilities that RFID technology has and that ten23 is on the forefront of innovation”, said Hanns-Christian Mahler, CEO of ten23 health.
“The BD iDFill™ RFID Integration Tour at CPHI Frankfurt offers a unique, hands-on experience to observe the full workflow in action—from filling to final assembly. It demonstrates how RFID-enabled prefillable syringes are now part of the existing ecosystem supporting efficiency, deviation management, and compliance.” Fernand Goldblat, VP/GM BD Medical-Pharmaceutical Systems, Prefillable Syringes Platform
“Manufacturers need greater efficiency, regulators demand enhanced traceability, and patients deserve certainty in product integrity. The CPHI demonstration shows how industry collaboration transforms unit-level traceability from concept to reality”– Thomas Hiltbrand, CEO and Founder, Crypto Pharma
The BD iDFill™ RFID Integration Tour is open to all CPHI 2025 attendees, with two small-group sessions per day (8–12 participants per tour, ~60 minutes). Visitors will move between four partner booths to follow an RFID-enabled syringe through key manufacturing steps: filling (Bausch + Ströbel), inspection (Körber), assembly (Harro Höfliger), and packaging with full unit-level data capture (ten23 health).
The collaboration builds on growing demand for digital traceability in pharma, driven by stricter regulatory expectations and the need for faster deviation management.
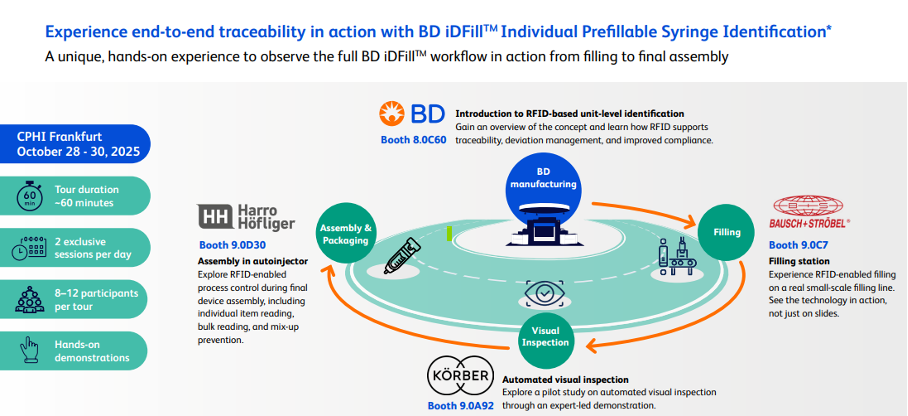
- BD uses RFID chips in the syringe lids (BD iDFill™) to ensure syringe traceability throughout every step of the filling process.
- ten23 health is a CDMO that develops, manufactures, and tests sterile medicines for the pharmaceutical industry and biotech start-ups.
- Harro Höflinger enables lab-scale assembly of injection devices using its semi-automatic Assembly Lab, supporting approval-phase manufacturing.
- Crypto Pharma increased manufacturing efficiency and ensures product integrity through innovative unit-level traceability solutions.
Link for a registration: BD iDFill Tour RFID Integration Tour – CPHI 2025

